- Secure payments
- In stock, ready to ship
- Backordered, shipping soon
Usually ready in 24 hours
Pickup available
Via Andria, 33
70033 CORATO BA
Italy
3293911307
COMPOSITION:
Active ingredient: Chewable tablets for dogs 2–4 kg: Afoxolaner (mg) 11.3.
Excipients: Corn starch, Purified soy proteins, Braised beef flavouring, Povidone (E1201), Macrogol 400, Macrogol 4000, Macrogol 15 hydroxystearate, Glycerol (E422), Medium chain triglycerides.
INDICATIONS:
• Treatment of flea infestations (Ctenocephalides felis and C. canis) in dogs, for at least 5
weeks. The product can be used as part of a treatment strategy for control
of flea allergy dermatitis (FAD).
• Treatment of tick infestations (Dermacentor reticulatus, Ixodes ricinus, Rhipicephalus sanguineus) in dogs. One treatment eliminates ticks for up to a month. In order to be exposed to the active ingredient, fleas and ticks must attach to the host and begin a blood meal.
• Treatment of demodicosis (caused by Demodex canis).
• Treatment of sarcoptic mange (caused by Sarcoptes scabiei var. canis).
CONTRAINDICATIONS/SIDE EFFECTS:
Do not use in cases of hypersensitivity to the active ingredient or to one of the excipients.
SAFETY IN THE REFERENCE SPECIES:
To allow parasites to be exposed to afoxolaner, they must initiate a blood meal on the host; therefore it is not possible to exclude the risk of transmission of diseases originating from the parasite.
SPECIAL PRECAUTIONS FOR USE:
Special precautions for use in animals In the absence of available data, the treatment of puppies less than 8 weeks of age and/or dogs weighing less than 2 kg should be based on a risk-benefit assessment by the veterinarian responsible. Special precautions to be taken by the person administering the veterinary medicinal product to animals To prevent children from accessing the veterinary medicinal product, remove only one tablet at a time from the blister. Place the blister with the remaining chewable tablets in the box. Wash your hands after handling the product.
PHARMACEUTICAL FORM:
Chewable tablets. Red to reddish brown, round shaped variegated tablets (tablets for dogs weighing 2 to 4 kg)
USE/ROUTE OF ADMINISTRATION:
For oral use.
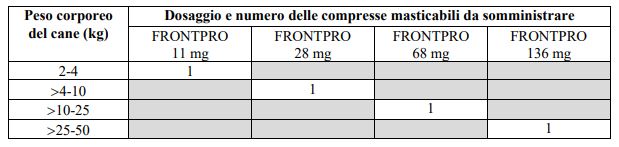
DOSAGE:
The product must be administered at doses of 2.7 to 7 mg/kg of body weight as per the following table:
For dogs weighing more than 50 kg, use an appropriate combination of chewable tablets with different/same dosages. The tablets should not be divided.
METHOD OF ADMINISTRATION:
The tablets are chewable and palatable for most dogs. It is possible to administer the tablets with food if the dog does not agree to take them directly.
TREATMENT PROGRAM:
Treatment of flea and tick infestations: Monthly intervals during the periods of greatest seasonality for fleas and/or ticks, based on the local epidemiological situation. Treatment of demodicosis (caused by Demodex canis): Continue monthly administration of the product until two negative skin scrapings are obtained one month apart. Severe cases may require extended monthly treatments. Since demodicosis is a multifactorial disease, where possible, it is advisable to adequately treat any other underlying pathology. Treatment of sarcoptic mange (caused by Sarcoptes scabiei var. canis): Monthly administration of the product for two consecutive months. Based on clinical evaluation and skin scrapings, it may be necessary to continue with further monthly administrations of the product.
TARGET SPECIES:
Dogs
ADVERSE REACTIONS (frequency and severity):
Mild gastrointestinal disorders (vomiting, diarrhoea), pruritus, lethargy, anorexia and neurological symptoms (convulsions, ataxia and muscle tremors) have been reported very rarely. Most reported adverse reactions were self-limiting and short-lived. The frequency of adverse reactions is defined using the following conventions: - very common (more than 1 in 10 treated animals exhibits adverse reactions) - common (more than 1 but less than 10 animals in 100 treated animals) - uncommon (more than 1 but less than 10 animals out of 1,000 animals treated) - rare (more than 1 but less than 10 animals out of 10,000 animals treated) - very rare (less than 1 animal out of 10,000 animals treated, including isolated reports).
OVERDOSE (symptoms, emergency procedure, antidotes):
No adverse reactions were noted in healthy Beagle puppies over 8 weeks of age treated with a dosage equal to 5 times the maximum dose repeated 6 times at intervals of 2 to 4 weeks.
WAITING TIME:
Not applicable.
INTERACTIONS:
None known.
DIAGNOSIS AND PRESCRIPTION:
Veterinary medicinal product without a veterinary prescription.
PREGNANCY AND BREASTFEEDING:
Laboratory studies performed in rats and rabbits have not shown the existence of teratogenic effects, or any adverse reactions on the reproductive capacity of male and female subjects. The safety of the veterinary medicinal product has not been established during pregnancy and lactation or in breeding dogs. Use only in accordance with the risk-benefit assessment of the responsible veterinarian.
SPECIAL STORAGE PRECAUTIONS:
This veterinary medicinal product does not require any special storage conditions.